
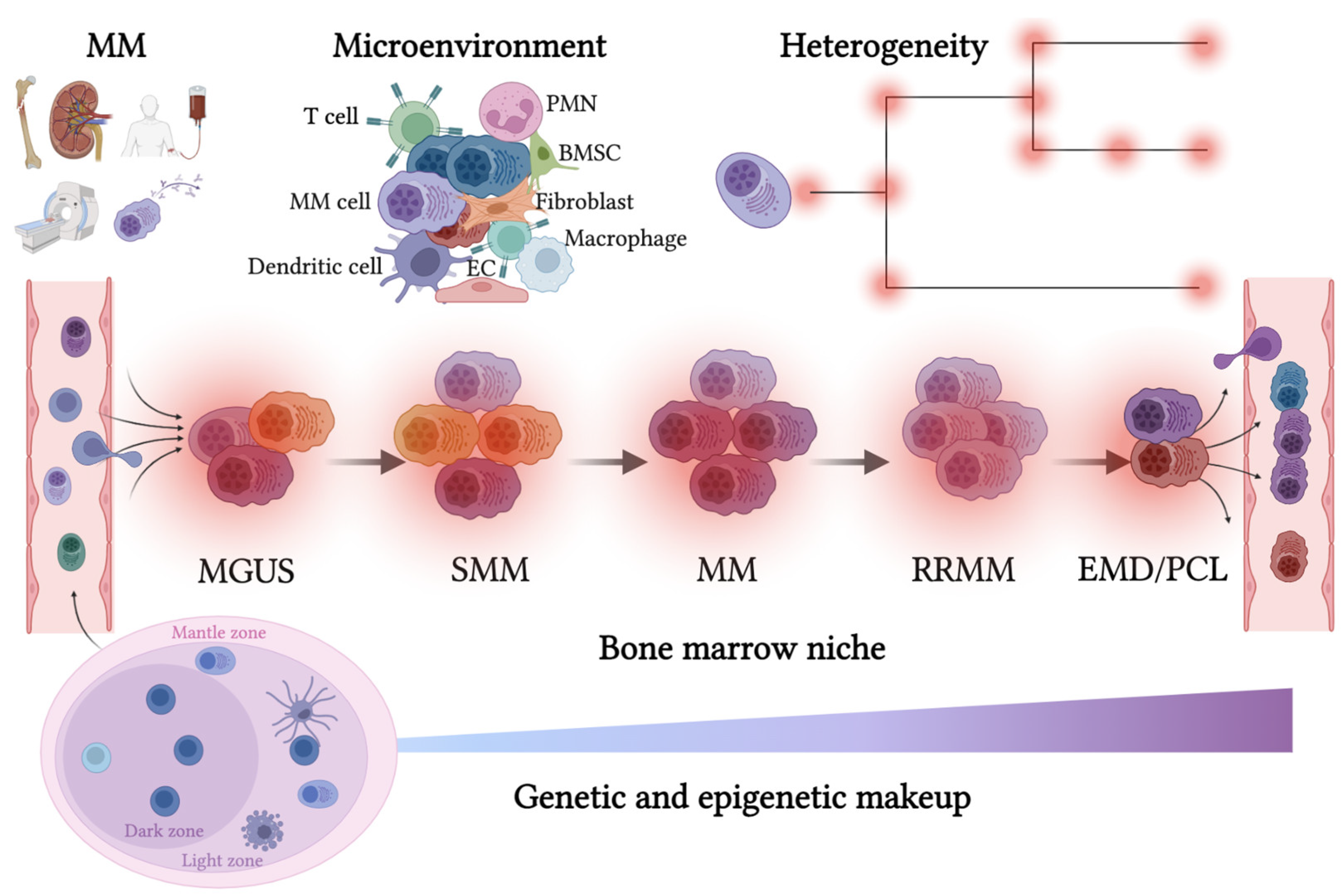
The historical lack of preclinical models reflecting the genetic heterogeneity of multiple myeloma (MM) hampers the advance of therapeutic discoveries. To circumvent this limitation, we screened mice engineered to carry eight MM lesions (NF-κB, KRAS, MYC, TP53, BCL2, cyclin D1, MMSET/NSD2 and c-MAF) combinatorially activated in B lymphocytes following T cell-driven immunization. Fifteen genetically diverse models developed bone marrow (BM) tumors fulfilling MM pathogenesis. Integrative analyses of ∼500 mice and ∼1,000 patients revealed a common MAPK–MYC genetic pathway that accelerated time to progression from precursor states across genetically heterogeneous MM. MYC-dependent time to progression conditioned immune evasion mechanisms that remodeled the BM microenvironment differently. Rapid MYC-driven progressors exhibited a high number of activated/exhausted CD8+ T cells with reduced immunosuppressive regulatory T (Treg) cells, while late MYC acquisition in slow progressors was associated with lower CD8+ T cell infiltration and more abundant Treg cells. Single-cell transcriptomics and functional assays defined a high ratio of CD8+ T cells versus Treg cells as a predictor of response to immune checkpoint blockade (ICB). In clinical series, high CD8+ T/Treg cell ratios underlie early progression in untreated smoldering MM, and correlated with early relapse in newly diagnosed patients with MM under Len/Dex therapy. In ICB-refractory MM models, increasing CD8+ T cell cytotoxicity or depleting Treg cells reversed immunotherapy resistance and yielded prolonged MM control. Our experimental models enable the correlation of MM genetic and immunological traits with preclinical therapy responses, which may inform the next-generation immunotherapy trials. New experimental models provide much-needed tools for understanding how genetically diverse multiple myeloma progresses and evolves in response to therapy.
The historical lack of preclinical models reflecting the genetic heterogeneity of multiple myeloma (MM) hampers the advance of therapeutic discoveries. To circumvent this limitation, we screened mice engineered to carry eight MM lesions (NF-κB, KRAS, MYC, TP53, BCL2, cyclin D1, MMSET/NSD2 and c-MAF) combinatorially activated in B lymphocytes following T cell-driven immunization. Fifteen genetically diverse models developed bone marrow (BM) tumors fulfilling MM pathogenesis. Integrative analyses of ∼500 mice and ∼1,000 patients revealed a common MAPK–MYC genetic pathway that accelerated time to progression from precursor states across genetically heterogeneous MM. MYC-dependent time to progression conditioned immune evasion mechanisms that remodeled the BM microenvironment differently. Rapid MYC-driven progressors exhibited a high number of activated/exhausted CD8+ T cells with reduced immunosuppressive regulatory T (Treg) cells, while late MYC acquisition in slow progressors was associated with lower CD8+ T cell infiltration and more abundant Treg cells. Single-cell transcriptomics and functional assays defined a high ratio of CD8+ T cells versus Treg cells as a predictor of response to immune checkpoint blockade (ICB). In clinical series, high CD8+ T/Treg cell ratios underlie early progression in untreated smoldering MM, and correlated with early relapse in newly diagnosed patients with MM under Len/Dex therapy. In ICB-refractory MM models, increasing CD8+ T cell cytotoxicity or depleting Treg cells reversed immunotherapy resistance and yielded prolonged MM control. Our experimental models enable the correlation of MM genetic and immunological traits with preclinical therapy responses, which may inform the next-generation immunotherapy trials. New experimental models provide much-needed tools for understanding how genetically diverse multiple myeloma progresses and evolves in response to therapy.

A future paradigm for precision immunotherapy, incorporating extensive
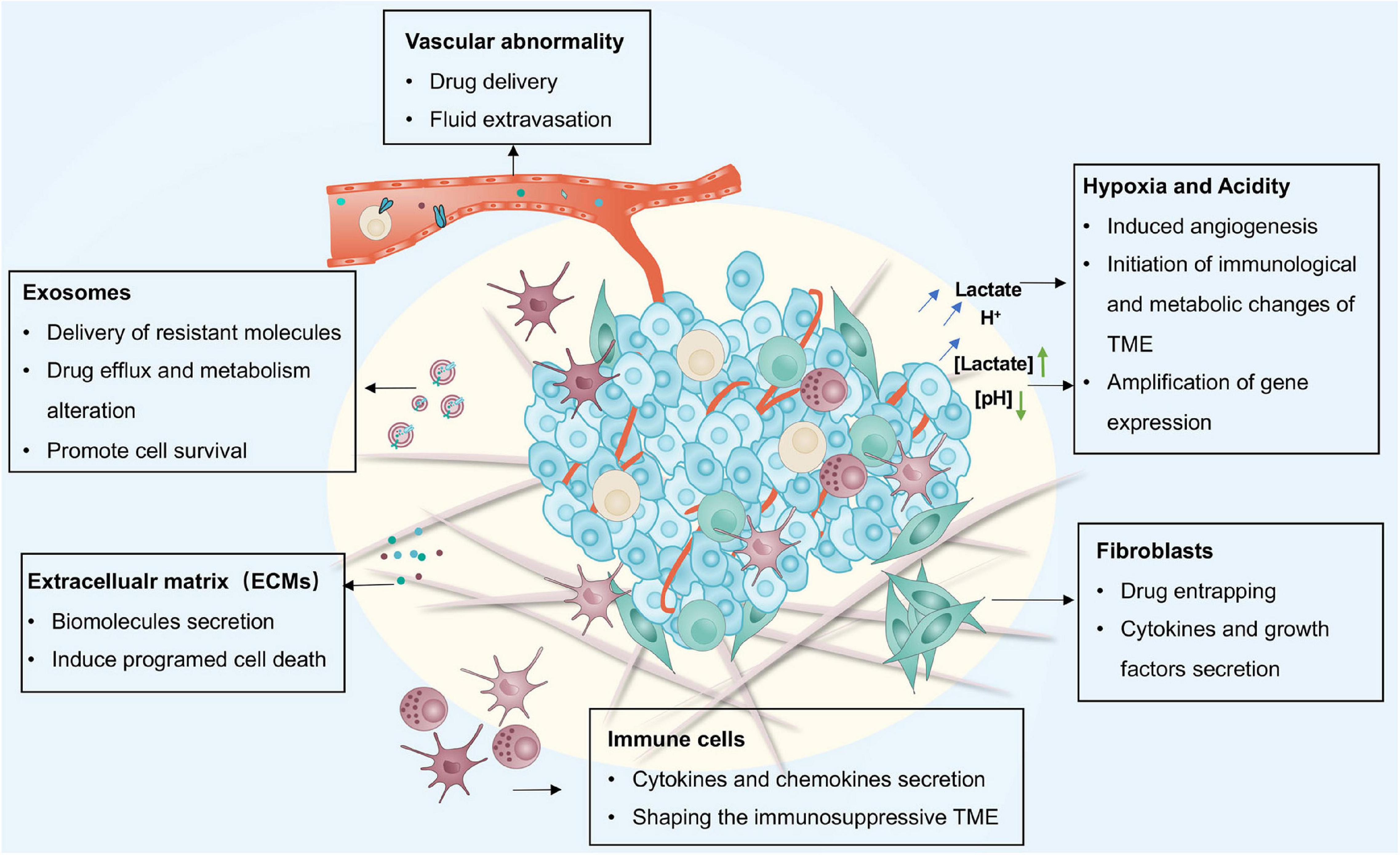
Frontiers Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment
Cancers, Free Full-Text
Frontiers Organoids: new frontiers in tumor immune microenvironment research

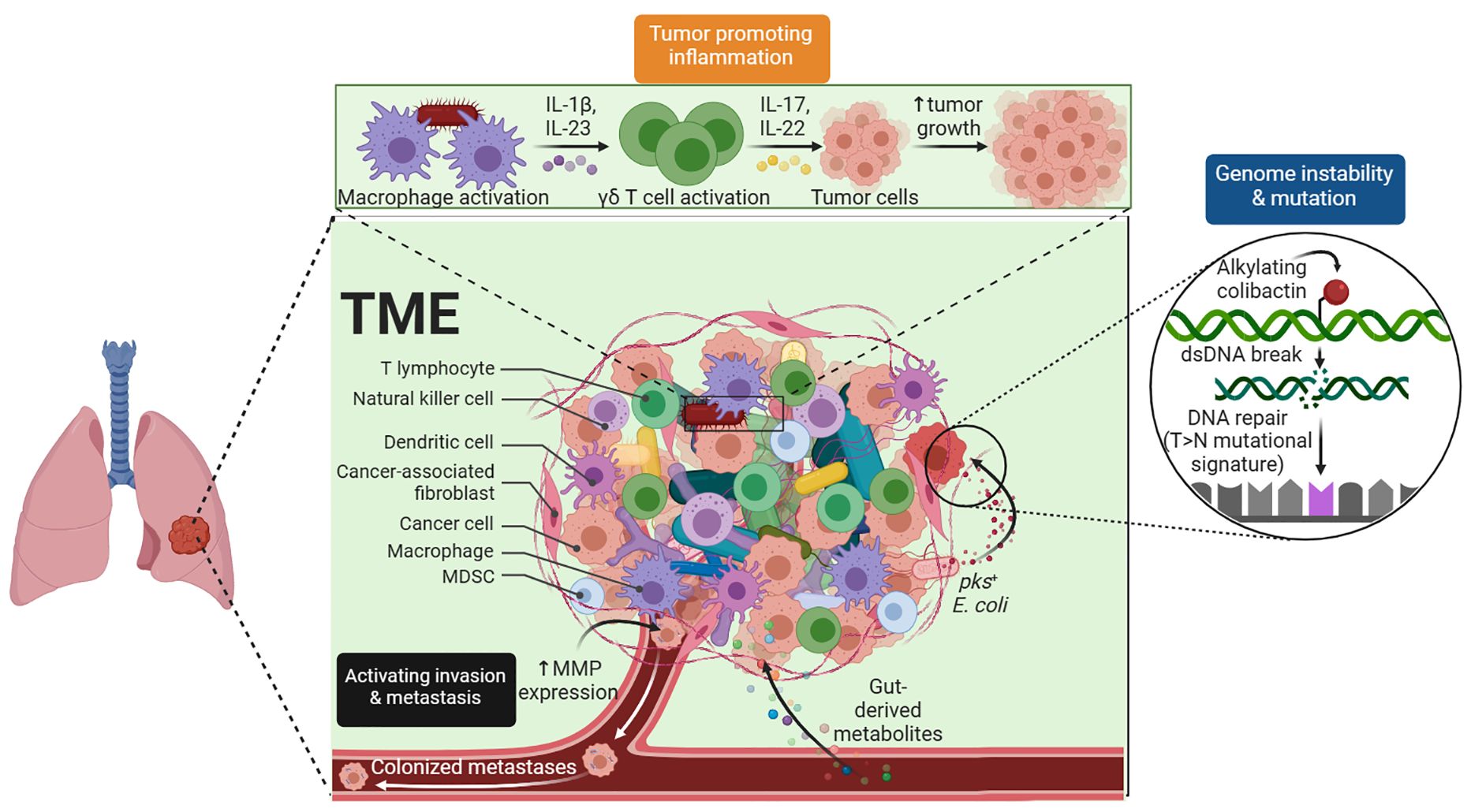
Immunotherapy approaches for hematological cancers - ScienceDirect

Modeling human tumor-immune environments in vivo for the preclinical assessment of immunotherapies

The cancer-immunity cycle: Indication, genotype, and immunotype - ScienceDirect
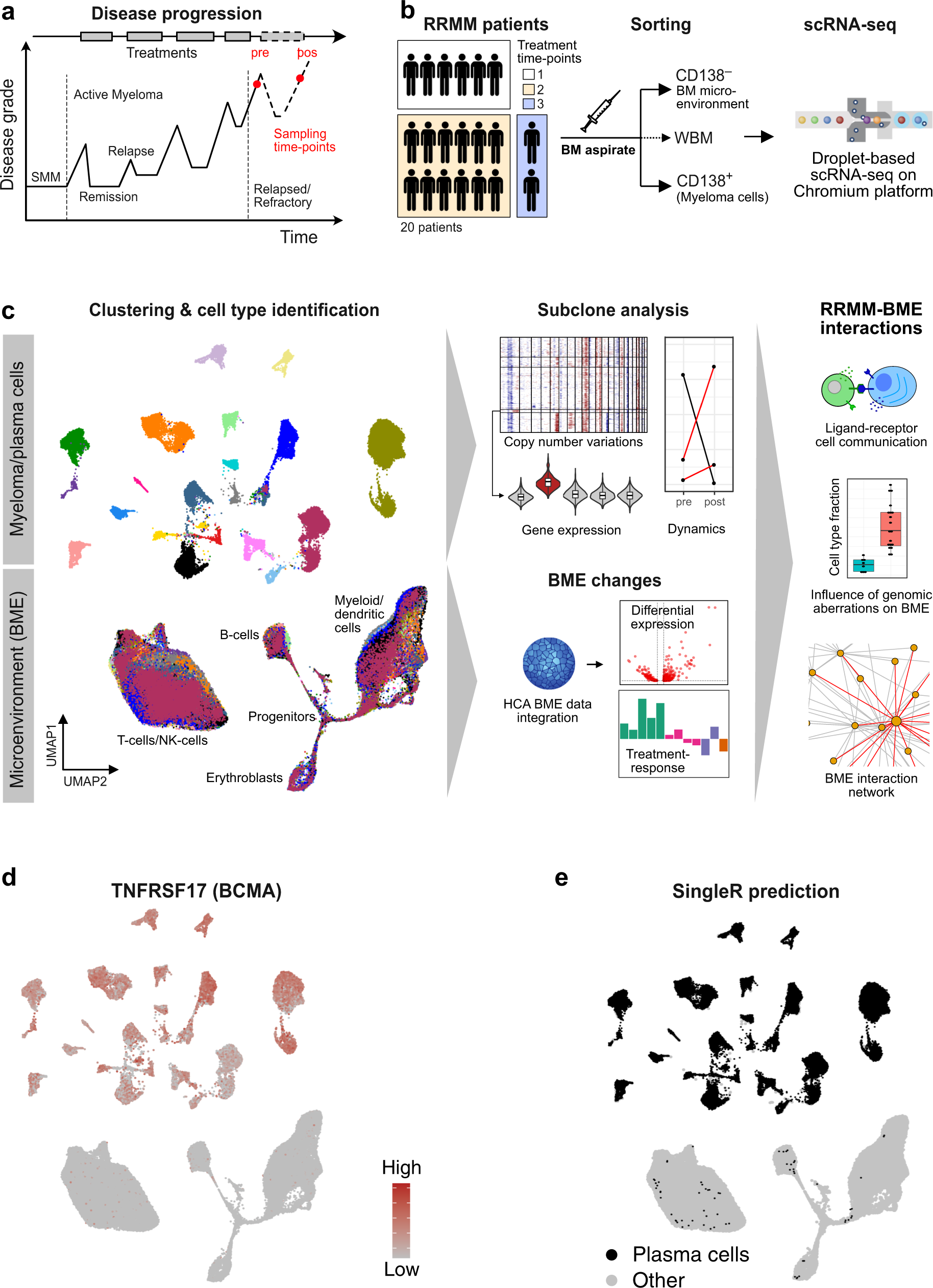
Subclone-specific microenvironmental impact and drug response in refractory multiple myeloma revealed by single‐cell transcriptomics
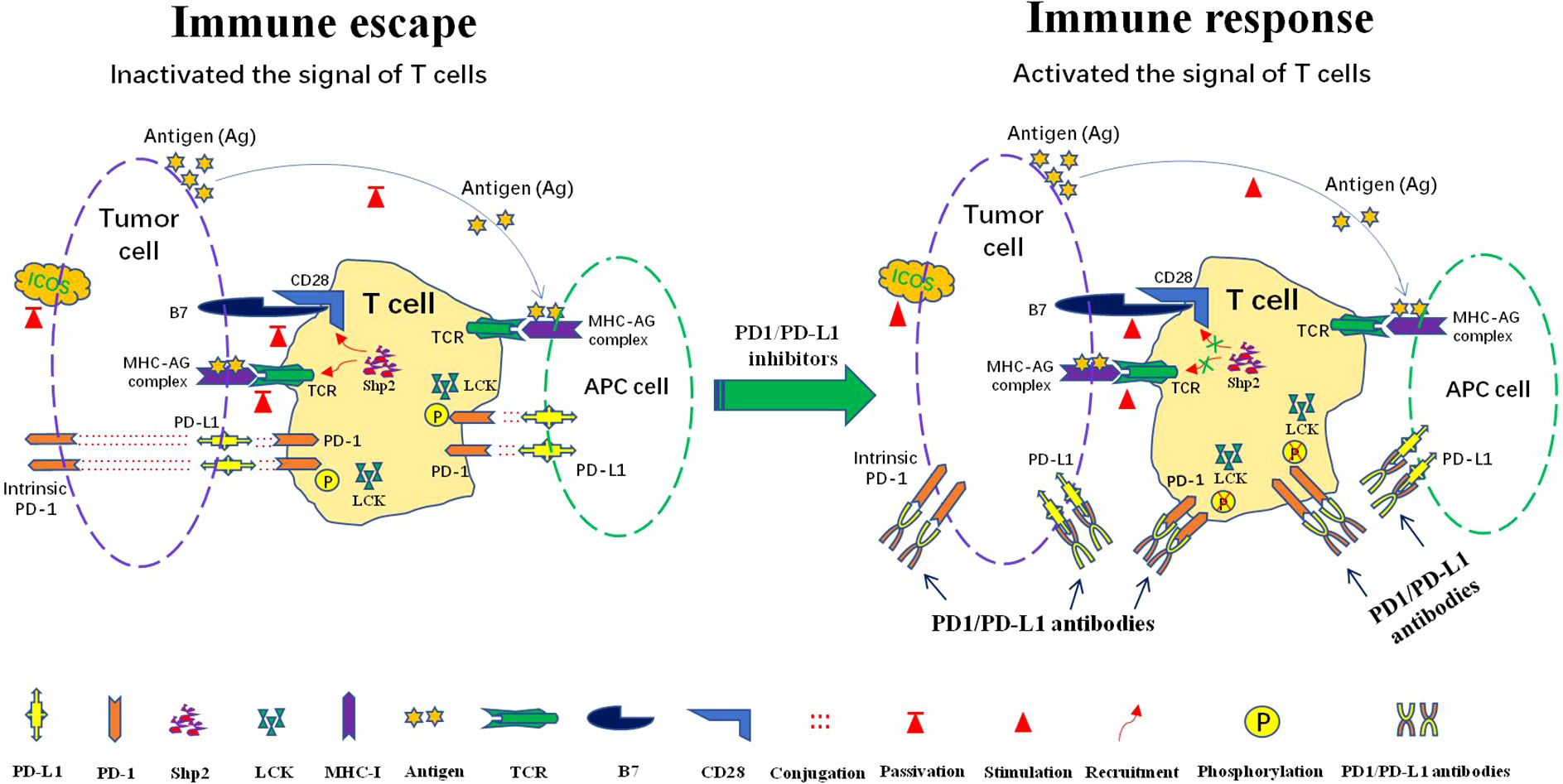
Frontiers The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers
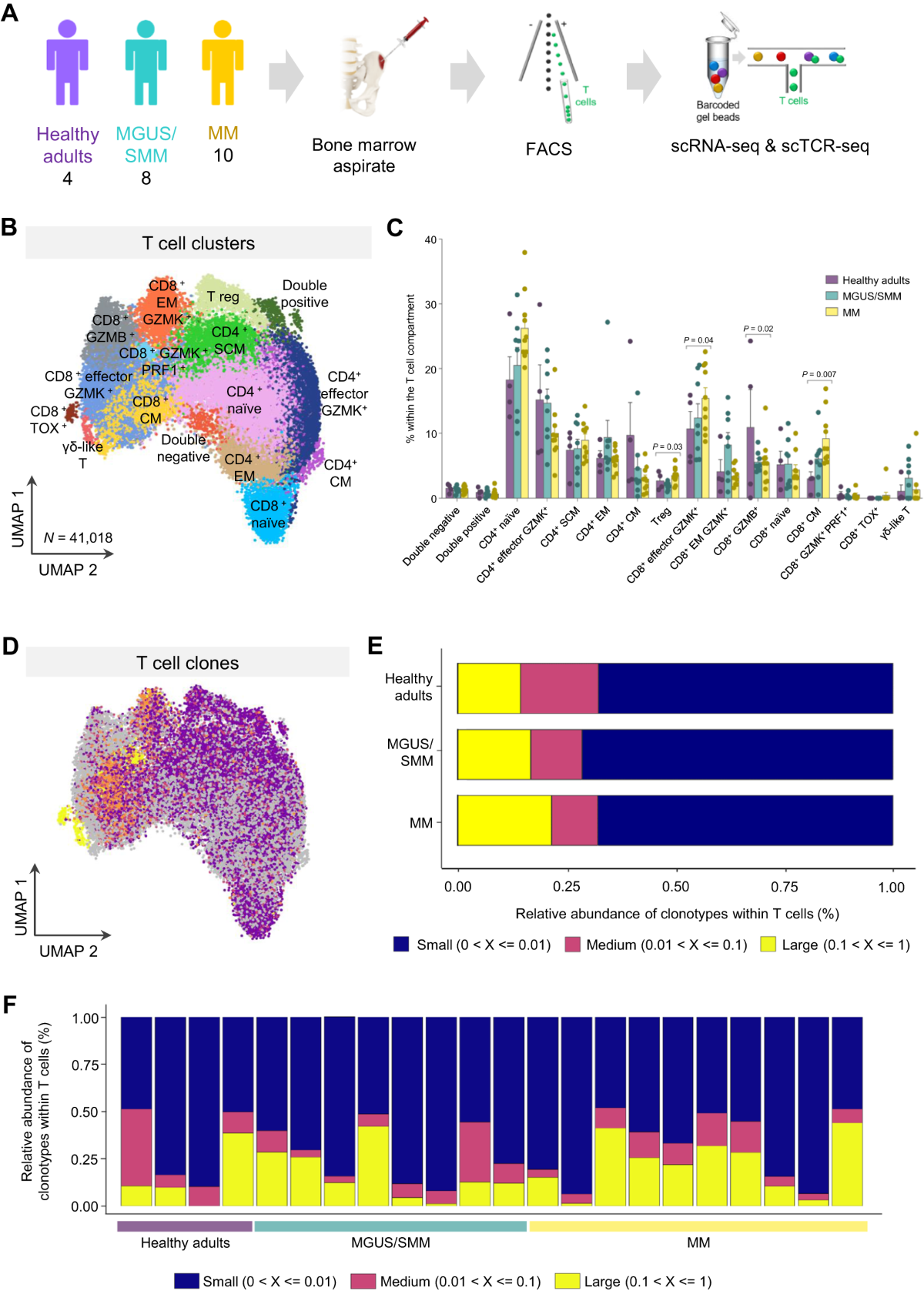
Large T cell clones expressing immune checkpoints increase during multiple myeloma evolution and predict treatment resistance
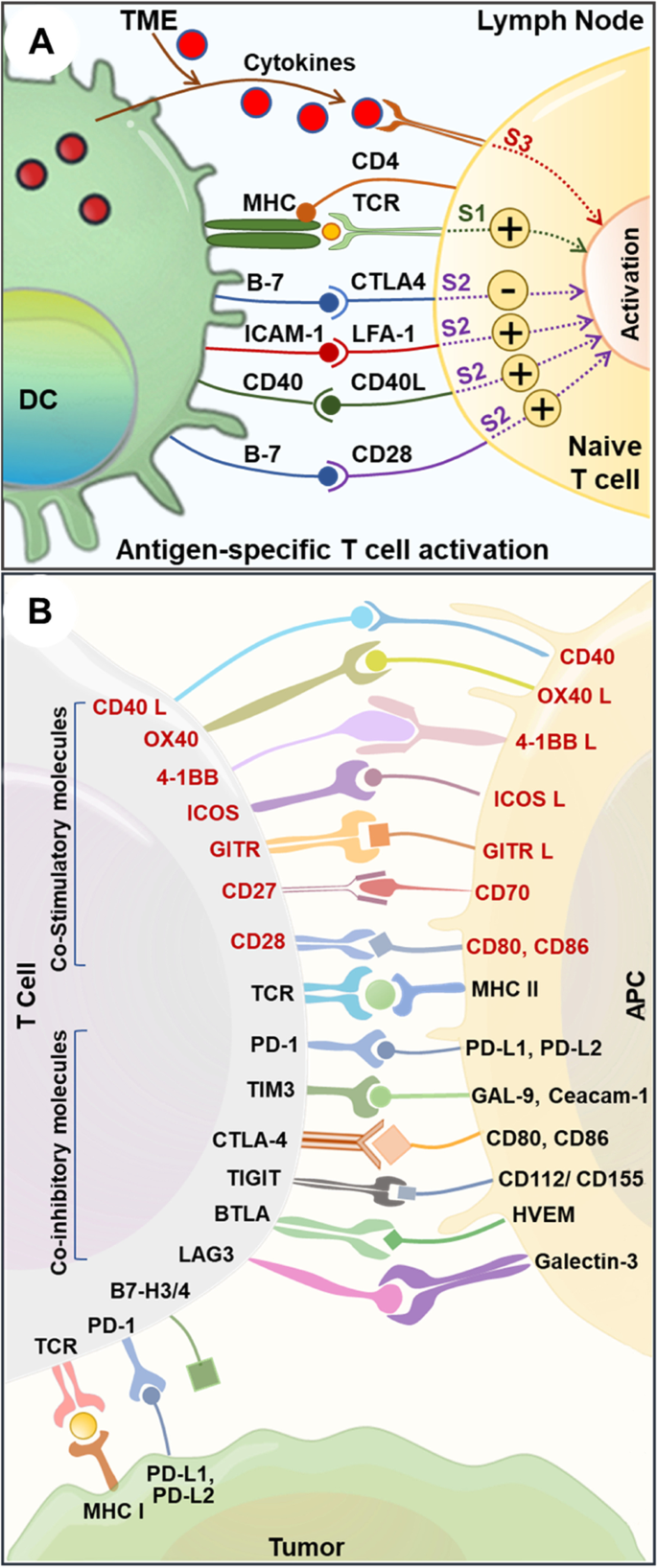
Lung cancer immunotherapy: progress, pitfalls, and promises, Molecular Cancer

Modeling human tumor-immune environments in vivo for the preclinical assessment of immunotherapies